You dropped a glass, and it came together again in your hands?
If you drop your glass of water and it shatters all over the floor, you’ll be a bit annoyed. But f it then spontaneously reassembles and jumps back up into your hand, you won’t be relieved to have your drink back. You’ll be thoroughly freaked out. Things like that just don’t happen.
This is why it’s usually easy to tell if a video recording is being played forwards or backwards. Smashed glasses reassembling, clouds of smoke rushing into narrow chimneys, pulped meat and bread emerging from mouths and assembling into pristine burgers – all of these tell us instantly that the video is being rewound. And if we were to see them in real life, we would know that time was running in reverse.
But if you were to look at a video recording made through a sufficiently powerful microscope, one that could see the basic interactions of particles that make up all these events, there would be no way to tell which was the forward playback and which was the reverse. Whether atom A knocks into atom B or vice versa, whether an atom loses energy by emitting a photon, or gains energy by absorbing one, all these processes are equally possible. The fundamental laws of physics – quantum field theory, general relativity – work equally well if time is reversed.
In theory, then, there is no reason why your dropped glass shouldn’t repair itself. All that needs to happen is that all the air molecules displaced by the broken pieces of glass should flow back and strike the fragments, giving just enough force to move them back the way they came, and similarly for all the drops of water, then for the glass molecules at the fractures to all move at the same time so as to repair the damage, enabling the glass to hold the water that is flowing back into it, and finally for the air molecules moved out of the way as the glass fell to move back and lift the glass back up into your hand. Cheers.
None of this would violate the microscopic laws of physics. So why doesn’t it happen? Why do we see such a difference between time going forward and backward?
Well, there is one set of physical laws that make a distinction between past and future. These are the laws of thermodynamics. In particular, the second law of thermodynamics states that, in an isolated system, entropy – a property related to energy and temperature – can increase or remain unchanged, but can never decrease. The significance of this is that entropy is a measure of the useful work that a system can do. The lower the entropy, the more capacity there is for the system to do work. As entropy increases, the system is less and less able to do anything.
For example, imagine you have an insulated box at -20 C – well below freezing point. You can use this to make ice cubes. Just fill an ice cube tray with water, pop it in the box, and wait for the water to freeze. Let’s assume this box is perfectly insulated, so heat doesn’t leak in through the walls, and you are able to open and close the door quickly enough that the inside doesn’t heat up while you’re doing it. Even so, this box is not going to be able to make ice cubes forever. Every tray of water you put in transfers some heat to the inside of the box, so every time you take a tray of ice cubes out the inside of the box will be slightly hotter. If you have a thermometer measuring the interior temperature, you will be able to see the temperature rising: -49, -48, -47… Even if you haven’t bothered with that, you will notice that each tray of water takes longer to freeze than the last one, as the temperature difference between the box and the water gets smaller.
Eventually, if you have a hell of a lot of gin and tonics to make and thus a massive demand for ice, you will notice that the water stops freezing at all, as the inside of the box has finally got above freezing point. Your box is now useless, and your gin and tonics will be sadly lacking. If you still have all the ice cubes around, you could try to cool the box back down by stuffing them back inside it, but at best you’ll manage to melt all your ice, get the box back to -20 C and be no better off than you were before you started. In fact, you probably won’t even be able to do this much. The ice will have warmed up and melted a bit while it was sitting around outside, and so some of your cooling power has been lost forever.
This is the remorseless march of entropy. As time goes on, temperature differences are smoothed out and thermal systems can do less and less work. Indeed, one of the many versions of the second law simply states that heat flows from higher temperatures to lower temperatures, but never the other way around.
At this point, some of you may be saying “But I have a box like that in my kitchen, and it provides me with ice for my G&Ts whenever I want!” Yes, but if you stick your hand round the back of it you will notice it’s warm on the outside. That’s because your freezer box is attached to an electric heat pump, and the entropy it generates by emitting heat into your kitchen allows it to maintain your ability to make ice.
The laws of thermodynamics were first codified during the industrial revolution in order to describe large-scale phenomena like hot gases pushing pistons – a vital application of science to emerging technology. As scientists began to realise that matter was made up of microscopic particles – atoms – the question arose of how the behaviour of these particles gave rise to the laws that govern the visible world. Eventually it would become clear that the laws of thermodynamics are about much more than steam engines and freezers: they are fundamental to the workings of the Universe, the nature of life, and the march of time itself.
The key to all this is a branch of physics called statistical mechanics. The idea behind this is that it is humanly impossible to calculate all the movements and collisions of the atoms even in something so simple as a box full of gas – and also pointless, as we can’t measure all these motions anyway. But by figuring out the average behaviour of the atoms, we can calculate quantities we can measure, such as pressure and temperature, and figure out how they change.
The second law of thermodynamics flows very naturally from this sort of calculation. Take a simple system, like the box of gas from the previous paragraph. As the atoms of gas move around inside the box, they randomly move through all the possible configurations of the system. These possible configurations could have all the atoms evenly distributed, or all of them concentrated into one corner, or all of them at the top of the box and none at the bottom, or whatever. They key point is that there are lots and lots of ways the gas can be evenly distributed, and relatively few ways it can be arranged in just one part of the box. So if the gas is already evenly distributed, it is overwhelmingly likely to stay that way, and if it is put into one of these special configurations it will quickly diffuse out and become evenly distributed once more.
It turns out that this is exactly equivalent to the second law of thermodynamics. The gas all in one corner is a low-entropy state, and the evenly distributed gas is a high-entropy state. The low-entropy system will spontaneously transform into the high-entropy system, while the high-entropy system will stay as it is.
But it also shows how the second law matters for any kind of physical system. A highly ordered system -say, a glass full of water – will readily become a less ordered system – say, a load of broken glass in a puddle – but not the other way around. There are many more ways for glass and water to be arranged in a mess on the floor than there are for them to be arranged as a container full of refreshing beverage.
The second law is also essential for life. As far as thermodynamics is concerned, life ingests low-entropy substances (high-frequency light, plants, animals), uses them to build up low-entropy structures (leaves, muscles, memories) and excretes high-entropy waste products (low-frequency light, poo, more poo). Ultimately, all life on Earth depends on the fact that the entropy of radiation is inversely proportional to its temperature. The Earth absorbs light from the Sun that has a temperature of 5500 C, and emits radiation at a temperature of about 20 C. The entropy difference is what powers all life on this planet.
So the arrow of time points from low entropy to high entropy. This implies that the Universe must have begun in a low-entropy state, from which it is gradually emerging as entropy rises. Is this true?
The further we look out into the Universe, the further back in time we are looking. Light from a galaxy a million light years away has taken a million years to reach us, which means we are seeing the galaxy now as it was a million years ago. So if it is really true that the thermodynamic arrow of time points in the direction of increasing entropy, then surely the further back we look into space, the lower the entropy should be.
But that’s not what we see at all. The oldest and most distant radiation we can detect is the cosmic microwave background radiation, or CMBR. Just after the Big Bang, all the matter in the Universe was in the form of a hot plasma, a gas made up of subatomic particles because it is too hot for atoms to survive. These days, space is cold and dark, with rock, gas and dust orbiting around the scattered stars and galaxies. This is because, as the Universe expanded, it cooled, and when the temperature had dropped enough the plasma formed into atoms of hydrogen, helium and other light elements. The time when this happened is called the recombination time, and it was about four hundred thousand years after the Big Bang – around thirteen billion years ago.
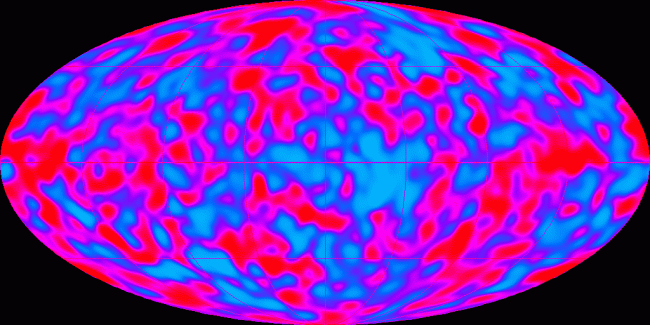
The CMBR is the light that was emitted by the hot plasma at the recombination time. It is the oldest light we can ever see. Looking back at the CMBR is like looking at the Sun – we can see the light given off at the surface, but nothing within it. Unlike the Sun’s light, the CMBR is primarily in the microwave part of the spectrum. It was visible light when it was emitted, but the expansion of the Universe in the intervening thirteen billion years has stretched out the light waves, shifting them to higher and higher wavelengths until now they are only detectable by very sensitive radio telescopes.
This radiation was first detected in 1965 by Penzias and Wilson – quite by accident, as an annoying hiss in their radio detection equipment – and was swiftly recognised as confirmation of the then-controversial Big Bang theory of cosmology. Perhaps an even more important and profound observation, however, was in 1992, when the COBE spacecraft mapped the structure of the entire CMBR, showing us not only a picture of the entire Universe in its primordial state, but also the tiny fluctuations in the plasma that would eventually grow into stars and galaxies, planets and people.
And it’s those tiny fluctuations that are the problem, as far as the thermodynamic arrow of time is concerned. They are a problem precisely because they are so tiny. The COBE observations showed that, thirteen billion years ago, the Universe was in a state of complete thermal equilibrium to within one part in one hundred thousand. This means the entire cosmos was at the same temperature, around 3000 degrees, with only minute variations.
The thing is, thermal equilibrium like this is a high-entropy state. The highest, in fact. All thermodynamic systems will tend towards thermal equilibrium, just as a ball on a hill tends towards rolling downwards. And when the system reaches thermal equilibrium, like the ball reaching the bottom of the hill, it stops.
So there’s a paradox. For time to work the way it does, the Universe must have started in a low-entropy state. But from the CMBR it looks like entropy in the early Universe was about as high as it can be. Does this mean all our ideas about time are wrong?
Not quite. We haven’t taken into account the fact that the Universe is expanding. This prevents the contents of the Universe from remaining in global thermal equilibrium, as the volume they occupy is increasing too quickly for them to be able to thermodynamically adjust. For an example of how this can work, consider a Universe consisting only of radiation and dust – a commonly-used model for the real Universe after the recombination time, when the CMBR was emitted. As the Universe expands, it cools. But these two components of the Universe cool at different rates. Matter cools more quickly than radiation. This creates a temperature difference, pulling the entire Universe out of thermal equilibrium. In other words, as the Universe expands, its entropy decreases.
So Vicki’s glass smashes because the Universe is expanding. Or to be a little more precise, a smashable object like Vicki’s glass only exists because the Universe is expanding. And the same goes for our experience of time, our laying down of memories, the measurable difference between the past and the future. All of these phenomena, however mundane, ultimately depend upon the structure of the Universe itself.
But what happens when all the glasses have smashed, when all the stars have burnt out, when entropy finally wins as the Second Law assures us it must? Will time come to an end?
It depends what you mean by time. However much the Universe expands, however old it gets, it will always be a four-dimensional structure with time as one of its coordinates. However, at some point in the far distant future, the subjective experience of time will indeed cease.
The scenario is this. Eventually all the stars will have collapsed into black holes, and these black holes will have absorbed all the other matter in the Universe. But that is not the end. Black holes eventually evaporate, shrinking as they give off radiation until they vanish entirely. So eventually there will be nothing left in the Universe except photons, particles of light. Now, according to the Special Theory of Relativity, for any particle moving at the speed of light every event is simultaneous. To a photon, there is no such thing as time. So when there is nothing left but photons, there will be nothing in the Universe that can experience time, nothing that can be aware of one event happening before or after another. Time will have come to an end.
And the Universe will carry on expanding. Forever.
Fantastic. I hope you have something in mind for “Logopolis” now. 🙂
Oh, I definitely have something in mind for Logopolis – though it may not be what you expect…